Abbey, GSK, Imena … all have actions! Multinational pharmaceutical companies seek "change" after the epidemic
Xi ‘an Jansen became history, Novartis spun off Sandoz, Pfizer Vaccine Division changed coaches, and Abbey’s position as the "king of medicine" was not guaranteed … Since September, many multinational pharmaceutical companies have experienced great changes from personnel to department structure to R&D pipeline.
Over the years, Greater China has been an important market component of many multinational pharmaceutical companies, and it is also the main driving force for their performance contribution. In the post-epidemic period, factors such as the difficulty in research and development of innovative drugs, declining performance, fierce competition in the same industry and continuous changes in industry policies are all affecting the strategic adjustment and personnel changes of multinational pharmaceutical companies in China. How to go in the second half of 2023? These pharmaceutical companies have taken the lead in making adjustments.
Johnson & Johnson: The History of Xi ‘an Janssen
The brand rejuvenation of the former giant Johnson & Johnson attracted the most attention. On September 14th, Johnson & Johnson announced that it would integrate its medical technology and pharmaceutical businesses into Johnson & Johnson’s name. Among them, Jansen changed its name to Johnson & Johnson Innovative Pharmaceutical, which also means that the logo of "Xi ‘an Jansen" will become history.
Once upon a time, the advertisement of Xi ‘an Jansen, "Stomach power is insufficient, please help with motilium", went deep into thousands of families in China, so that its Dakening, Sismin, Caile, Tylenol … all became necessities at home. The history of Xi ‘an Jansen dates back to 1961, when the Belgian scientist Dr. Paul Jansen joined Johnson & Johnson with his Janssen Pharmaceutical Companies. Since then, the strength of the Johnson & Johnson pharmaceutical sector has been greatly enhanced, and the research and development of prescription drugs has also been on the right track. However, after 2002, the OTC products that Xi ‘an Jansen relied on for a living began to decline. The fundamental reason was that the original star products, Motilium and Dyclonine, were already in the decline period of product life cycle, and the follow-up new products were unsustainable. Johnson & Johnson Group never gave Xi ‘an Jansen any new product support.
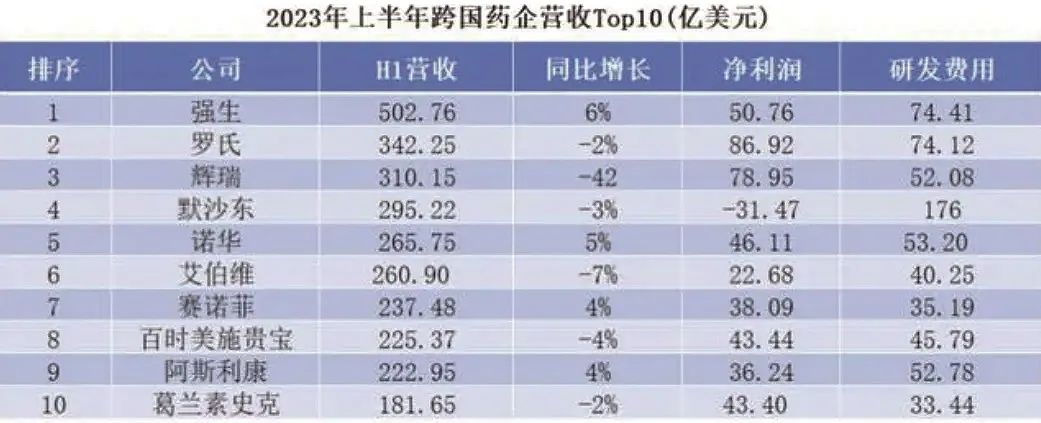
In 2021, under the pressure of cost, Johnson & Johnson began to carry out drastic reforms, divesting the consumer health business and listing it independently, becoming a dual-business company spanning the two major sectors of medical equipment and medicine. This series of reforms has also kept its performance growing in recent years. The semi-annual report in 2023 showed that its total revenue exceeded the $50 billion mark, among which the revenue of pharmaceutical business in the first half of the year increased by 3.7% year-on-year to $27.144 billion. Immunity and tumor were the main sources of performance in Johnson & Johnson’s pharmaceutical business.
In the future, Johnson & Johnson will focus more on the fields of tumor, immunity, nerve, cardiovascular, pulmonary hypertension and retina, and develop cutting-edge drugs. In terms of personnel structure, Joseph Wolk, chief financial officer of Johnson & Johnson, said last year that due to economic pressure and the separation plan of consumer health business, Johnson & Johnson may moderately reduce the number of employees.
Pfizer: structural adjustment
Since September, the structure of Pfizer China’s Hospital Emergency Division and Vaccine Division, as well as the changes of relevant responsible persons, have come into effect. Among them, the head of the Vaccine Division changed from Zhang Lingyan to Yang Bei, and there are North China District, South China District and Market Platform under the charge of Hao Yikai, Shi Yinli and Jin Xinqing respectively. It is understood that although Yang Bei is the new leader of the Vaccine Division, she has been working in Pfizer for 25 years, and the other three responsible persons have been in Pfizer for more than ten years.
Some insiders speculate that the coaching change of Pfizer Vaccine Division may be related to the performance pressure of its vaccine products. According to Pfizer’s forecast, the revenue of its two major COVID-19 products, vaccine (Comirnaty) and oral medicine (Paxlovid), will be about $13.5 billion and $8 billion in 2023, respectively, down by 64% and 58% compared with 2022, and the gross profit of COVID-19 vaccine will be equally divided with BioNTech.
At the same time, another star product of Pfizer, 13-price pneumonia vaccine Pei Er 13, successfully expanded its age in April this year, but with the catch-up of domestic pharmaceutical companies, Pei Er 13 is no longer the only choice in the China market. In 2020 and 2021, watson biological’s Woanxin and Minhai Bio’s Weimin Feibao were listed successively, and the prices were 598 yuan/dose and 458 yuan/dose respectively, which were significantly lower than Pei Er’s 698 yuan/dose. And as early as before Pei Er’s 13th birthday, the vaccination targets of Woanxin and Weimin Feibao were infants and children from 6 weeks to 5 years old (before the 6th birthday).
However, judging from the coaching change, Pfizer remains optimistic about the vaccine field and the China market. According to the data of the semi-annual report, Pfizer increased the cost of vaccine product research and development by 530 million US dollars in the first half of this year. As of June this year, Pfizer has 12 vaccines under research, and 1/3 of them have entered Phase III clinical practice.
Lilly: preparing for diet pills
On October 1st, Yuan Ping, the current acting head of Lilly’s Diabetes Alliance Division, was appointed as the national executive sales director of Diabetes Portfolio Division, and the head of Diabetes Alliance Division was replaced by Liu Aihua, the current head of Cancer Portfolio Division. This change may be a warm-up for the launch and promotion of the slimming and hypoglycemic drug telpotide in the second half of this year.
The semi-annual report shows that Lilly’s total revenue in the first half of the year was 15.272 billion US dollars, up about 7% year-on-year; The net profit was US$ 3.108 billion, up about 9% year-on-year, of which Telpotide achieved sales of US$ 980 million. Not only has the performance increased significantly, but under the expectation of diet pills and new drugs for Alzheimer’s disease, as of September 25th, Lilly’s total market value has reached as high as $524.3 billion, with a share price of $522. Telpotide, like Novo Nordisk’s Smegrupeptide and Liraglutide, belongs to the most popular GLP-1 drugs at present. The indications for weight loss of Lilly Telpotide injection were declared and listed in China in August, and it is expected to be listed at the end of the year.
Abbey: Looking for the Next "King of Medicine"
Recently, AbbVie terminated two agreements with innovative pharmaceutical companies in China. Tianjing Bio announced on September 22nd that AbbVie had terminated the agreement with lemzoparlimab, a candidate drug for CD47 antibody jointly developed and sold by the company in 2020, and the termination agreement will take effect on November 20th this year. It is understood that CD47 is another hot target after PD-1. Another project that was terminated was an authorized cooperation between AbbVie and Jiakesi Pharmaceutical, a domestic biopharmaceutical company, on SHP2 inhibitors. Garcos announced the termination of the project in July this year, and within 180 days of handover, AbbVie will continue to reimburse all expenses under the pre-approved development plan.
Many people were surprised by AbbVie’s decision to give up two innovative drugs from China. Some insiders speculate that it is because clinical trials have not reached expectations. In order to save costs, it is reasonable for AbbVie to give up these two projects.
In the past 20 years, Hummel, the "drug king", has brought more than $200 billion in revenue to AbbVie. However, at the beginning of this year, Amjevita, the first bio-similar drug of Hummel, officially entered the American market. In addition, nine bio-similar drugs competed with Hummel, resulting in a decline in its sales. In the first quarter of this year, AbbVie’s revenue was US$ 12.225 billion, down over 8% year-on-year, while Hummel’s revenue was US$ 3.541 billion, down over 20% year-on-year. AbbVie previously predicted that the franchise of Hummel will be eroded by about 45% in 2023, and AbbVie, who is eager to find the next "drug king", has been forced to terminate the research and development of seven ADC drugs.
Roche: Layout "New Troika"
Chen Shaofeng, the head of Roche’s specialty medicine field (ophthalmology, neuroscience, anti-infection and immunity) in China, resigned at the end of July. Since August 31st, Chen Kaijuan, the head of global integration strategy of Sufuda in Roche headquarters, has taken over the position of head of specialty medicine field, reporting directly to Bian Xin, president of Roche China.
Since last year, Roche has continuously optimized its strategic product portfolio in China. With the patent protection of "Troika" of Avastin, Herceptin and Rituximab expiring one after another, Roche is laying out a new "Troika", that is, expanding from a single tumor field to three major fields of "tumor, neuroscience and ophthalmology".
To this end, Roche has announced an additional investment of nearly 250 million yuan in China as working capital. Including this investment, Roche has invested a total of 1.4 billion yuan in the China market in three years. At present, Roche’s investment has achieved initial results. According to the data of the semi-annual report, Roche’s total business income was 22.681 billion Swiss francs (about 181.624 billion yuan), an increase of 8% year-on-year; Among them, the pharmaceutical business income in China was 1.505 billion Swiss francs (about RMB 12.052 billion), up 3% year-on-year, accounting for 6.6% of Roche’s global pharmaceutical business income.
Sanofi: Stick to the "Slimming Plan"
On August 31st, Sanofi, a French pharmaceutical giant, announced that Bill Hibbard, the head of its global specialty drugs, had resigned. On September 18th, Sanofi sold its 11 central nervous system products to Pharmanovia, another multinational pharmaceutical company.
In the past two years, Sanofi has been actively "slimming" by selling consumer health care brands and 17 drugs in the fields of central nervous system diseases and vascular diseases. This series of "sale plans" has been advocated by Paul Hudson, the current CEO of Sanofi, since he took office. In 2019, he put forward the "play to win" plan, aiming to focus Sanofi’s business on key research and development fields such as immune inflammation, rare diseases, tumors and vaccines from 2020 to 2025.
At present, the slimming plan has not changed. As of the first half of this year, Sanofi has at least 78 clinical projects, most of which are concentrated in the above key areas. According to the semi-annual report, Sanofi’s sales in the first half of this year reached 20.187 billion euros (about 156.389 billion yuan), a year-on-year increase of 2%. Among them, the core product, that is, Duplex monoclonal antibody in the self-exemption field, sold 4.878 billion euros (about 37.77 billion yuan). Two new drugs approved last year: Enjaymo, which is used to reduce the demand for red blood cell transfusion caused by hemolysis in adults with cold agglutinin’s disease (CAD), and Xenpozyme, which is used in children and adult patients with acid sphingomyelinase deficiency (ASMD), have achieved an increase in volume, with an increase of 1800% and 750% respectively in the first half of the year. It can be seen that after getting rid of the non-main business, Sanofi has played a "home court advantage" more easily.
Novartis: Spinning off Generic Drug Subsidiary
On September 15th, Novartis announced that on October 4th, Sandoz, a subsidiary of Novartis, which specializes in generic drugs and bio-similar drugs, was officially split.
It took Novartis nearly five years to divest Sandoz. The initial split news came out in 2018, when Sandoz’s performance showed a downward trend and dragged down Novartis. Although Sandoz, Tiwa and Huizhi dominate the world’s top three in the field of generic drugs, Sandoz’s generic drugs brought Novartis only one-fifth of the total revenue in 2022, and the rest were created by innovative drugs. The data shows that in 2022, the revenue of Novartis innovative drugs was 41.296 billion US dollars, accounting for 82% of its total revenue; Sandoz’s revenue is $9.249 billion.
In order to become a truly innovative drug company, Novartis has been split up in recent years. In addition to Sandoz, Novartis also split Alcon, one of the largest ophthalmic products and equipment companies. In addition, in the field of treatment, Novartis has also focused on cardiovascular, kidney and metabolism, immunity, neuroscience and tumor from more than 10 original concerns.
At present, Novartis is moving towards the goal of being the largest innovative pharmaceutical company in the world. In the first half of this year, Novartis’s net sales were US$ 26.575 billion (about RMB 194.314 billion), a year-on-year increase of 5%; The net profit was US$ 4.611 billion (about RMB 33.715 billion), a year-on-year increase of 32%.
Immena: poor performance for "veteran"
Inmina, the leader in gene sequencing, announced on September 20th that Li Qing, the company’s global senior vice president and general manager of Greater China, had left. The day before, Waters announced the appointment of Li Qing as the company’s vice president and general manager of Greater China.
Imina is a global leader in gene sequencing and chip technology, and its product line covers scientific analysis instruments such as sequencing platforms, chip scanners and in-vitro diagnostic sequencers. But in recent years, the performance in China market is not good. In the fourth quarter of 2022, the revenue of Mina China dropped by 22%, and in the first quarter of 2023, the revenue dropped by 28% to 91 million US dollars (about 665 million yuan). According to this year’s semi-annual report, in the second quarter, Mina’s performance in China was still declining, and its revenue was US$ 115 million (about RMB 841 million), down 3% year-on-year.
In this regard, Immena explained that global inflation, exchange rate, slowing economic growth, and market competition have caused poor performance. Now that "veteran" Li Qing has gone, the challenge is still there, because it is still unknown whether Mina’s performance will pick up in the second half of the year.
GSK: R&D has been frustrated repeatedly
Recently, GSK announced that Dr. John Lepore, Senior Vice President and Director of R&D, had left the company. Dr. Kaivan Khavandi, the new R&D director who will succeed him, will lead GSK’s restructured respiratory and immunology department. On September 11th, WestlyYu, the current vice president of GSK China and head of respiratory business, was appointed as vice president and head of special medicine business, and continued to be one of the leading teams of GSK China, reporting directly to Qi Xin, general manager of GSK China. There are indications that the GSK changes not only personnel, but also internal structure and research and development direction.
In recent years, GSK has had a hard time, especially in the research and development of new drugs with high investment and high risk. In November 2022, an ADC drug under GSK was stopped by the FDA; In February of this year, the CEO of GSK announced that he would end all investment in gene therapy; In addition, GSK’s PD-1 drug Jemperli, which was acquired by acquiring Tesaro for $5.1 billion, sold only $8 million in the first half of 2022, far below GSK’s expectations.
GSK’s executive adjustment may be to change the status quo of making ends meet. GSK divides the R&D department into three groups: respiratory and immunology, vaccines and infectious diseases, and oncology, which are dedicated to initial discovery and early clinical research. GSK also said that it will not reduce investment in research and development, and will accelerate the development of products in the future.
AstraZeneca: Increase investment in China.
According to the British "Daily Mail" reported on September 10th, the CEO of AstraZeneca may leave as soon as next year. Although changes are on the way, AstraZeneca’s development in China is becoming more and more "localized". In 2023, AstraZeneca celebrated its 30th anniversary in China. Over the past 30 years, the China market has become increasingly important in AstraZeneca’s global strategic position, with R&D investment in China reaching 1.5 billion US dollars and investment exceeding 1 billion US dollars. At present, China has developed into AstraZeneca’s second largest market in the world, and is gradually changing from the original "main sales place" to "main production place" and "main creation place".
Following the formal signing of the domestic production and supply base of Budigefu inhalation aerosol with a total investment of about 450 million US dollars with Qingdao High-tech Zone on March 25th this year, AstraZeneca planned to invest another 250 million US dollars in Qingdao inhalation aerosol production and supply base project on August 14th, in order to increase the canning production capacity and build an inhalation aerosol packaging production line, and further expand the production and supply base capacity. In addition, it is reported that AstraZeneca will also set up a research and development center in Hong Kong.
Bayer and Bojian: A wave of layoffs is coming.
On September 15th, it was reported in Reuters that Bill Anderson, CEO of Bayer, was considering cutting management, and the specific plan will be put forward at the recent internal strategy meeting. On the same day, Oliver Kohlhaas, head of Bayer strategy, announced his resignation.
Although Bayer’s global spokesman declined to comment, Bayer’s layoffs have already begun. In February of this year, Bayer launched the "Resignation Option Plan" in California, USA, which laid off 55 employees who were over 55 years old and had served for 10 years. In August, BlueRock Therapeutics, a cell therapy company owned by Bayer, announced that it would lay off about 50 employees. In addition, since 2023, Bayer’s share price has only increased by 6%, far less than its peers, and Novartis’s share price has increased by 18% in the same period. It is reported that in the next few months, Bayer will formulate a more official comprehensive restructuring plan to boost the company’s share price, and many investors hope that it can split the two major departments of agriculture and medicine and go public independently.
Bayer is not the only one to reduce costs and increase efficiency. In July, Bojian, who just made a major breakthrough in the field of drugs for Alzheimer’s Harmo, announced that it would try to save $1 billion in operating expenses by 2025 by laying off 1,000 people and stopping the research and development of at least four drugs, and spend $300 million of it on the research and development of new drugs.
Survival, operational efficiency and clinical demand have always been the three driving forces for the continuous reform of domestic and foreign pharmaceutical companies. The reform and adjustment of multinational pharmaceutical companies are also inseparable from these three factors. In addition, in the face of a more complex and huge China market, it will take more effort. How innovative drugs can enter medical insurance, how to promote them after entering medical insurance, and how to compete with similar products have forced multinational pharmaceutical companies to make constant adjustments in structural changes, internal resources and key teams.
At present, the policy that has the greatest impact on the performance of pharmaceutical companies in the second half of the year is undoubtedly the medical anti-corruption action that is expected to last for one year. Tang Aijin, chief analyst of Cinda Securities Medicine, believes that medical anti-corruption will affect the sales rhythm in the short term, but it will not be broken. Strengthening the restriction and supervision of the whole industry will benefit the long-term healthy development of the industry, and pharmaceutical companies will pay more attention to R&D and innovation after the sales expenses are limited.